Revolutionising clinical trials: One2Treat’s patient-centric approach to drug development
Interview with Sebastien Coppe, chief executive officer and Pascal Piedbois chief medical officer, One2Treat
Estimated reading time: 9 minutes

Name:
CEO:
No of staff:
Company status: Head office: Sector:
Founded:
One2Treat
Sebastien Coppe
Twelve
Private
Belgium
Pharma R&D
2023
Who are One2Treat?
Founded in 2023 by Marc Buyse, One2Treat has emerged as a pioneering tech company dedicated to transforming clinical trial design through patient-centric innovation. Leveraging the successes of the two previous companies founded by Marc Buyse, IDDI and CluePoints, One2Treat has positioned itself at the intersection of technology, medical research and patient experience. One2Treat’s core mission is to develop sophisticated software solutions that enable pharmaceutical sponsors to design clinical trials that more authentically reflect patient perspectives and expectations, with a particular emphasis on rare diseases.
It’s innovative One2Treat Voice application is a tool that allows stakeholders to prioritise outcomes that truly matter, ultimately seeking to make clinical trials more meaningful, efficient and aligned with patient experiences. By bridging the gap between drug development, regulatory requirements and patient perspectives, One2Treat is not just developing software, but pioneering a paradigm shift in how medical treatments are conceptualised, developed and evaluated.
The broken paradigm of traditional clinical trials
For decades, clinical trials have followed a rigid, one-dimensional approach that often neglects the most critical stakeholders: patients themselves. One2Treat is dismantling this outdated model, introducing a novel methodology that places patient experience at the core of medical research. Led by Sebastien Coppe, CEO, and Pascal Piedbois as its CMO, the company aims to challenge the traditional approach introducing a multi-dimensional methodology that captures the nuanced perspectives of patients, clinicians and researchers.
Pascal describes what he sees as the fundamental flaw in existing clinical trial designs: “Traditionally, we had to decide on one primary endpoint. If you meet the primary endpoint, it’s a positive study. If you don’t, it’s a negative study.” This approach reduces complex medical treatments to a binary success-or-failure metric, overlooking the nuanced realities of patient experience.
Pascal recounts a conversation with a melanoma patient who emphasised the importance of stress levels—an outcome rarely considered in clinical trial design. “How about my level of stress taken as an example of outcome?” the patient asked, revealing a critical gap in traditional research methodologies.
Instead of relying on a single outcome and ignoring the things that matter to patients as per the example of the significance of stress, One2Treat’s approach considers multiple patient-reported outcomes, creating a more holistic understanding of treatment effectiveness.
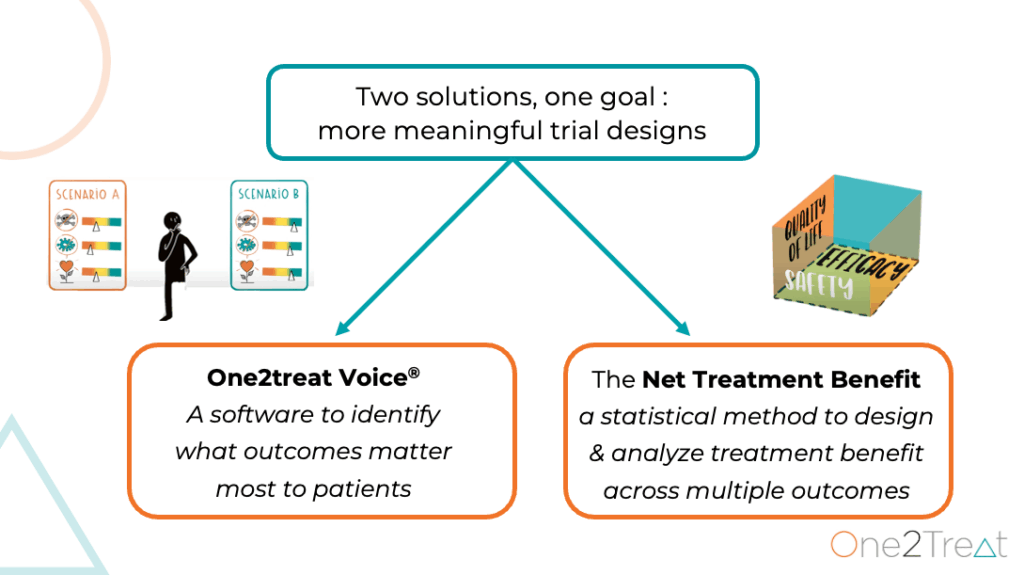
Leveraging smart technology: One2Treat Voice
At the core of One2Treats offering is a unique interactive application that captures patient and clinician preferences. Sebastien explains the tool: “What you would receive is two virtual patients with multiple potential outcomes, and you are asked to tell the app which patient has the best overall outcome. It is simple, intuitive and accessible.”
The application operates through a simple yet sophisticated comparative methodology:
- 1. Virtual patient scenarios
Users are presented with two virtual patients, each representing different treatment outcomes. Sebastien expands: “You see two virtual patients with various preselected outcomes and values attributed for each of those outcomes.”
- 2. Comparative decision-making
Participants are asked to select which virtual patient has the best overall outcome. For example, in an oncology trial, this might involve comparing overall survival times, adverse event occurrences, quality of life indicators and treatment burden.
- 3. Iterative comparison process
The application uses a repeated comparison method. As Sebastien describes, “You engage with the application for around just 10 minutes, clicking which patient has the best overall outcome.” Each selection provides insights into the user’s prioritisation of different treatment outcomes.
The application is designed to be inclusive and can be used by patients and patient advocates, clinical investigators, healthcare professionals and sponsors. They are also committed to adding caregivers as a future expansion, in recognition of the vast insight’s caregivers hold the key to, as Pascal expands.
“Caregivers have been ignored for so long in clinical research, yet they often shield the most significant burden of treatment. They witness the day-to-day realities that patients experience—the treatment complexities, the emotional challenges, the practical impacts that never appear in clinical data. Our vision is to create a methodology that doesn’t just listen to patients but truly understands the entire ecosystem of care. Caregivers can provide unique insights into treatment burden, quality of life, and the real-world implications of medical interventions that clinical trials traditionally overlook.”
By bringing multiple perspectives into its application, One2Treat are aiming to truly humanise medical research.”
It was important to One2Treat that the application itself was not burdensome, so it is developed with user experience in mind to ensure optimum participation and accessibility. It takes less that 10 minutes to complete and requires no specialised medical knowledge. Its intuitive and user-friendly interface allows participates to access it via any smart phone.
The detail in the data
One2Treat Voice goes beyond simple preference selection. It captures outcome prioritisation, individual thresholds of clinical benefit, individual decision-making processes and a nuanced understanding of treatment value based on individual participant’s insights. Pascal explains, “As an example; used in the context of paediatric epilepsy the app might compare scenarios involving; number of seizures, cognitive development, quality of life indicators and treatment side effects.”
One2Treat Voice then uses its analytical capabilities to provide sophisticated analysis of the data collected. This then generates individual preference mapping, aggregate outcome prioritisation and sensitivity analysis of different outcomes and their weightings.
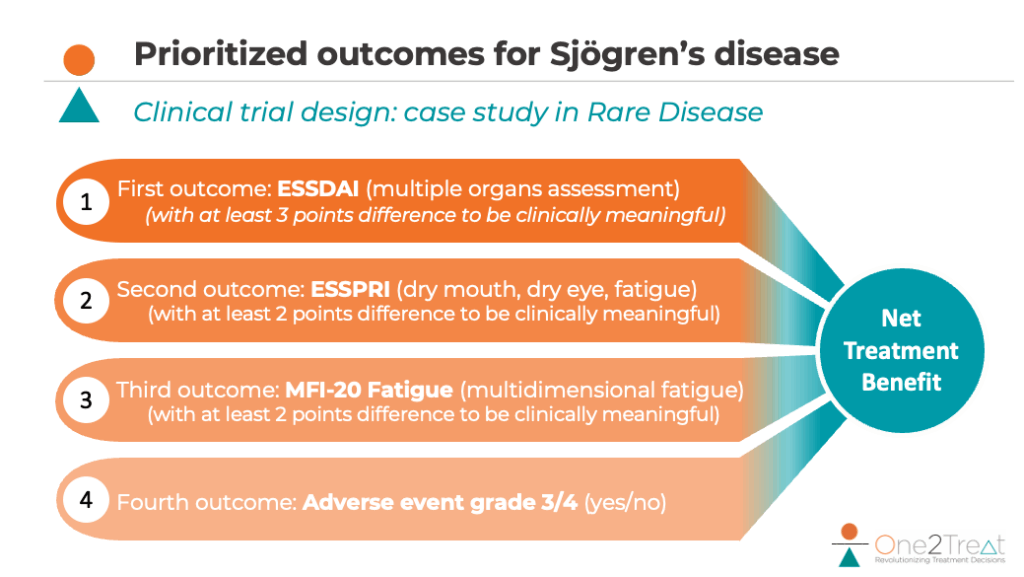
Data weighting and prioritisation
One2Treat’s methodology introduces a sophisticated approach to data weighting that goes beyond traditional clinical trial analysis. Pascal provides critical insights into how different stakeholder inputs are considered and integrated. “The weighting process is not a simple mathematical equation, but a nuanced dialogue between multiple perspectives. Patients provide fundamental insights into what matters most in treatment outcomes. Their input is crucial but not exclusively determinative. Site investigators and clinicians offer professional medical perspectives, adding depth to patient-reported outcomes.” Pharmaceutical sponsors must ultimately create a design that can be approved by regulatory agencies, so their insights also matter.
Pascal describes this as a “complex decision-making process”. “At the end of the day, you need to come up with one primary analysis. You have to come up with one statistical plan, and you have to make sure that this statistical analysis plan will be approved by the agency.”
Sebastien adds the importance of understanding threshold values. “For example, in an oncology context: Overall survival might be prioritised. But only if there’s a meaningful benefit (e.g., more than six months). If the benefit is marginal, other factors like quality of life become more important.” The analysis methodology of One2Treat Voice allows all of these nuanced factors to be considered.
Satisfying regulators: clinical trial validation
The intricate balance between innovative research methodologies and regulatory requirements lies at the heart of One2Treat’s approach. Far from being a challenge, the company views regulatory engagement as a critical opportunity to demonstrate the value of its patient-centric methodology. Pascal emphasises that the ultimate goal is “not just to collect data, but to create a compelling narrative that resonates with health and regulatory authorities”. This multi-outcome approach provides regulators with a more comprehensive view of a treatment’s potential value. By incorporating patient, clinician and sponsor perspectives, One2Treat creates a robust framework that goes beyond traditional single-endpoint clinical trials.
The health technology assessment advantage
A further critical benefit emerges in the realm of health technology assessment (HTA). Pascal explains that their method provides a “quantitative and global assessment of both efficacy and safety, which is precisely what regulatory bodies seek”. This approach reconciles drug development with market access, addressing a long-standing challenge in pharmaceutical research.
One of the most innovative aspects of their approach is the sensitivity analysis. By demonstrating how different outcome prioritisations impact the overall assessment, One2Treat provides regulators with unprecedented transparency. This method allows for a more nuanced evaluation of a treatment’s potential, going beyond the pass-or-fail binary of traditional clinical trials.
Addressing rare disease complexities
In rare diseases, where traditional trial designs often fall short, this approach becomes particularly powerful. Sebastien highlights that this methodology is particularly problematic in rare diseases, where a single outcome cannot adequately represent patient needs. The current system essentially ignores largely the nuanced experiences of rare disease patients meaning clinical trials often miss crucial insights that matter most to patients.
One2Treat sees rare diseases as a critical focal point for its work. Sebastien emphasises: “In rare diseases, you cannot easily find a single outcome that will reflect well what matters to patients. Moreover, by integrating several outcomes into one primary analysis, it’s often possible to reduce the required sample size for clinical trials by over 30%.” The company believes rare disease research demands a more nuanced understanding, where patient perspectives become paramount. Its methodology addresses two critical challenges: helping pharma sponsors design more meaningful trials and ensuring patient recruitment in conditions with limited patient populations.
By supporting reduced sample sizes and creating more patient-focused trial designs, One2Treat aims to make clinical research more efficient and representative. As Pascal notes, their goal is to “answer both pharma perspectives and patient perspectives,” with rare diseases representing the most compelling arena for this patient-centric approach. The company’s commitment extends beyond data collection, seeking to create trials that genuinely reflect the lived experiences of patients in these challenging and often overlooked medical conditions.
Future regulatory landscape
One2Treat sees its approach as more than a current solution—it’s a potential paradigm shift in how clinical trials are conceived and evaluated. By demonstrating the value of a more comprehensive approach, they hope to influence regulatory thinking in the long term.
One2Treat sees its approach as a sophisticated evolution in clinical trial design—not just satisfying current requirements—reshaping the future of medical research validation. It’s a bold vision and undertaking that both Sebastien, Pascal and the One2Treat team embrace fully.
Connect with Sebastian
Connect with Pascal
in the spotlight profiles innovative companies working in the RARE space. To access more in the spotlight articles click below.


